
8442
Large-scale studies are an important chapter in the development of a cardiac drug. For example, 8442 patients and more than 1000 medical centers in 47 countries, such as the Papa Giovanni XXIII Hospital in Bergamo (photo), participated in a long-term heart failure study.
Published on 28/02/2022
Text by Michael Mildner, Photos by Laurids Jensen




The aging of society is a major reason for the high incidence of heart failure disease. At the beginning of this millennium, patients still faced a difficult future. Despite therapeutic advances in improving blood flow and the control of blood pressure, the chances of survival were low. On average half of all patients died within five years of a heart failure diagnosis.

In Italy, more than 200 patients participated in the Novartis study, including many at Papa Giovanni XXIII Hospital in Bergamo. Among the study participants was Teresa Benedetti, who had been suffering for years from heart failure caused by high blood pressure, and in 2002 received a pacemaker which had to be replaced seven years later. She was full of energy during our 2016 visit to Bergamo.
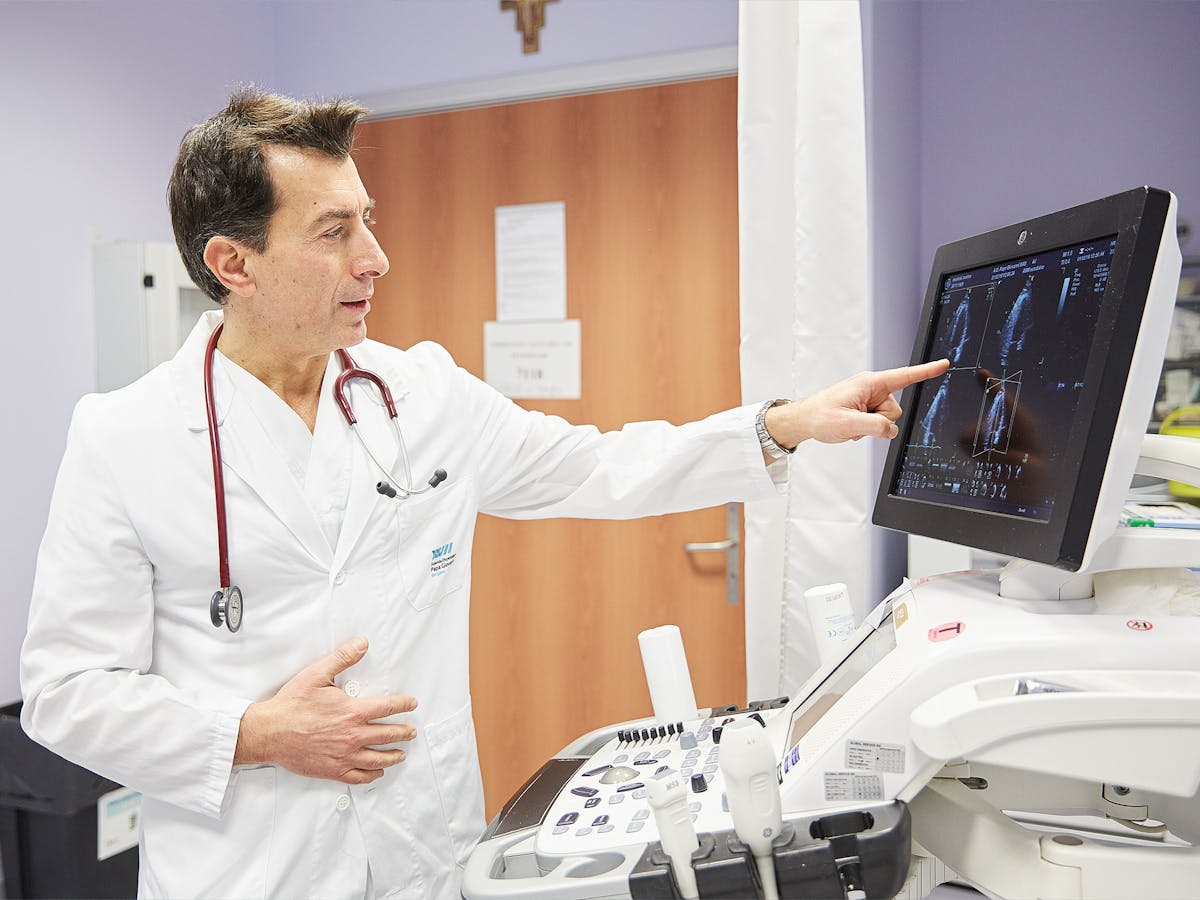
The examinations at Bergamo Hospital were carried out by numerous highly specialized medical professionals, such as co-study leader Dr. Vincenzo Duino. Among other things, he checked the patients’ cardiac data and took blood samples. Not only the number of study participants, but also the application for approval of the cardiac drug Entresto was gigantic: 87.1 gigabytes of data containing more than 4 million pages were submitted to the U.S. Food and Drug Administration in 2014. The application also contained 211 592 pages of laboratory data, physician protocols, drug analyses and much other information.

8442
Large-scale studies are an important chapter in the development of a cardiac drug. For example, 8442 patients and more than 1000 medical centers in 47 countries, such as the Papa Giovanni XXIII Hospital in Bergamo (photo), participated in a long-term heart failure study.
Published on 28/02/2022
Text by Michael Mildner, Photos by Laurids Jensen




The aging of society is a major reason for the high incidence of heart failure disease. At the beginning of this millennium, patients still faced a difficult future. Despite therapeutic advances in improving blood flow and the control of blood pressure, the chances of survival were low. On average half of all patients died within five years of a heart failure diagnosis.

In Italy, more than 200 patients participated in the Novartis study, including many at Papa Giovanni XXIII Hospital in Bergamo. Among the study participants was Teresa Benedetti, who had been suffering for years from heart failure caused by high blood pressure, and in 2002 received a pacemaker which had to be replaced seven years later. She was full of energy during our 2016 visit to Bergamo.

The examinations at Bergamo Hospital were carried out by numerous highly specialized medical professionals, such as co-study leader Dr. Vincenzo Duino. Among other things, he checked the patients’ cardiac data and took blood samples. Not only the number of study participants, but also the application for approval of the cardiac drug Entresto was gigantic: 87.1 gigabytes of data containing more than 4 million pages were submitted to the U.S. Food and Drug Administration in 2014. The application also contained 211 592 pages of laboratory data, physician protocols, drug analyses and much other information.





