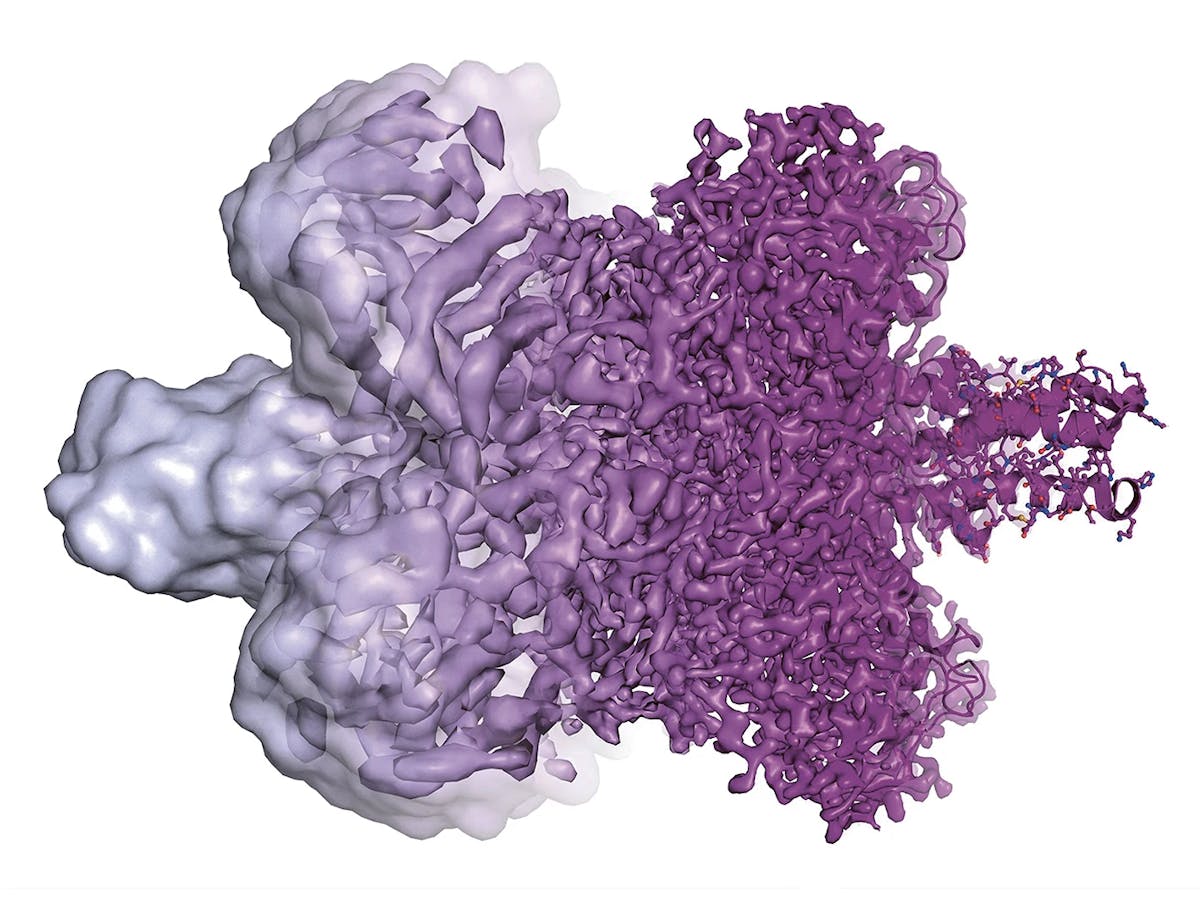
Researchers from Novartis and the FMI prepare a protein sample, which is later analyzed with the help of the cryo-electron microscope.
Published on 02/11/2020
It was a great day for science and a proud moment for Switzerland when Jacques Dubochet from the small town of Morges received the Nobel Prize in chemistry in 2017.
Together with Joachim Frank and Richard Henderson, with whom he shared the award, the retired professor from the University of Lausanne had provided the groundbreaking research that gave rise to cryo-electron microscopy.
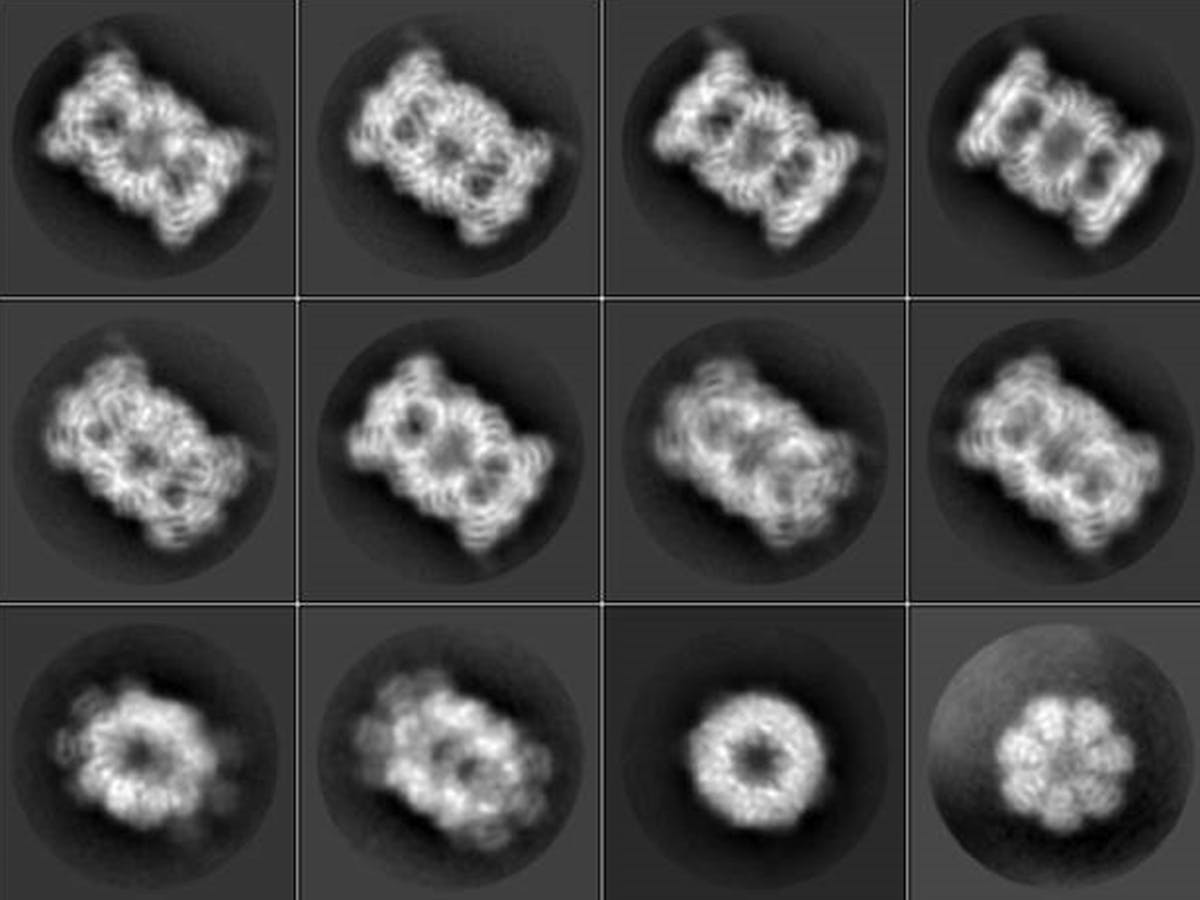
Cryo-EM, as it is known for short, is a new imaging technology which allows stunning views of complex molecular structures such as proteins.
The Nobel Prize for Dubochet and his colleagues had been expected for a couple of years already because the image technology had leapfrogged to previously unknown resolution levels within a short period of time. Only 10 years ago, images of proteins developed with the help of a cryo-electron microscope were blurred at best and were laughingly referred to by insiders as “blobs.” But then, rapid advances in digital technology and much improved cameras helped create 3D images of breathtaking sharpness, revealing nature’s beauty in hitherto unthinkable clarity.
Gold standard techniques such as protein crystallography and nuclear magnetic resonance spectroscopy almost paled in comparison.
Scientists around the world were aflame with excitement.