
Shaping drug production
Once a new drug candidate has been identified, the Technical Research & Development (TRD) group at Novartis evolves the final drug presentation. Moving the project from Chemical to Pharmaceutical Development and to Quality Control, TRD’s task is to hand over a robust process to the commercial production facilities. Although this sounds like a standard task in the pharmaceutical industry, every drug has its own challenges to overcome along the way.
Text by Michael Mildner, Photos by Laurids Jensen and Bjoern Myhre, videos by Nicolas Heitz.








The synthesis steps of a new drug candidate are executed in the labs of the Chemical & Analytical Development group, which is part of TRD.
In this case, it takes nine synthesis steps before the final drug molecule is available. The laboratory flask holds a volume of 300 milliliters ready to be handed over to the ensuing analysis and purification processes.
Samples of the synthesized product are, among others, tested for enzyme residues. The device used for this analysis works like a sophisticated microwave with temperatures up to 165°C, pressure and acids.
Once the synthesis is complete, the product is tested for various aspects.
The high-performance liquid chromatography (HPLC) equipment is used to separate, identify and quantify each component in the synthesized product. It enables the TRD scientists to identify all contents and any impurities that might occur.
This modified and upgraded HPLC equipment is combined with a small reactor inside, which also allows toxicological characteristics of samples to be identified.
In addition to the high-tech equipment, traditional work in the laboratory fume hood is also in demand.
Samples of the synthesized product are subject to additional tests in addition to automated devices. Ultimately, accomplishing the task requires significant human interaction and teamwork.
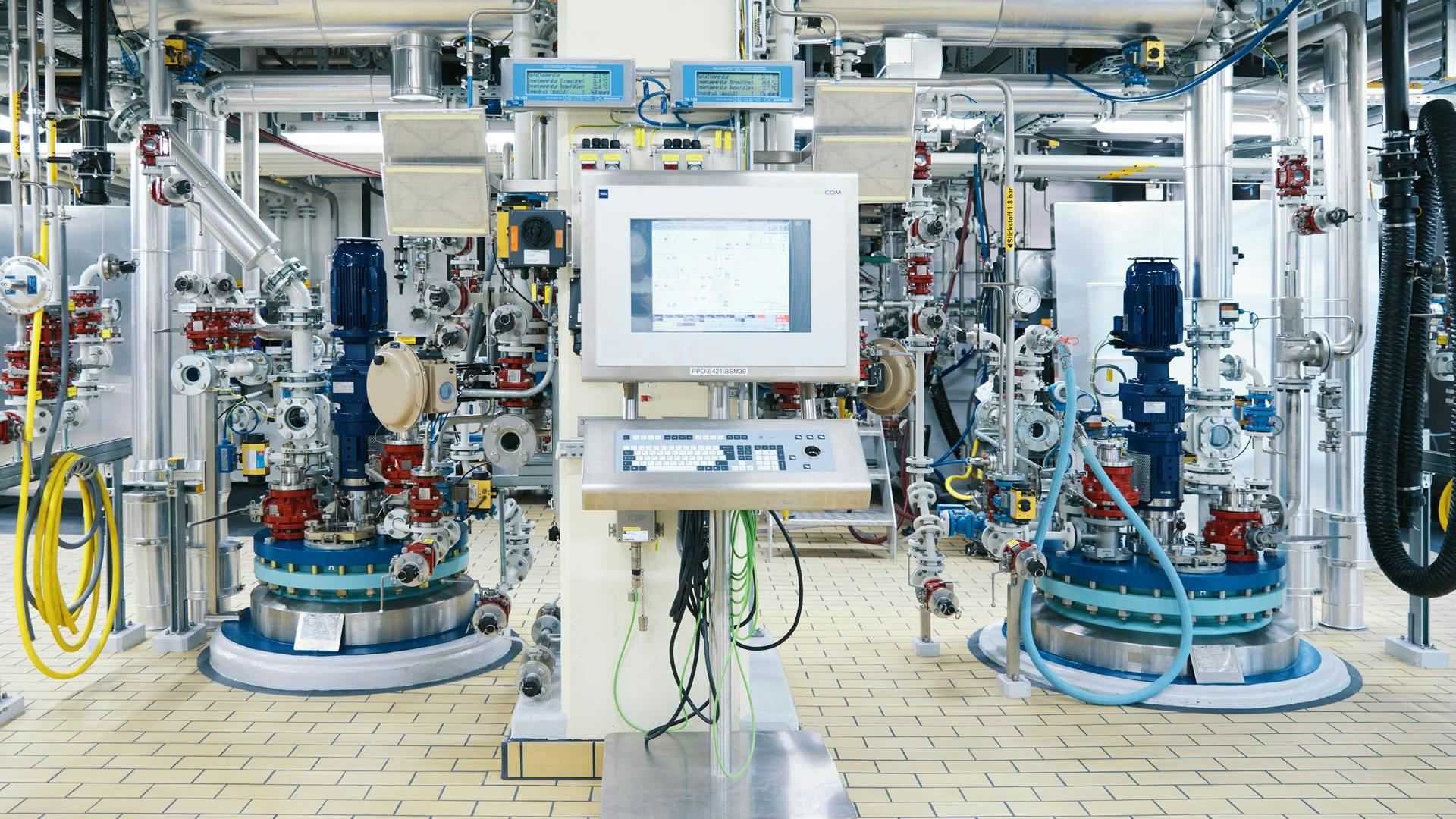
The drug substance production for clinical studies happens in Building 145 on the Novartis Campus.
The building’s reactors are spread over multiple floors and operated mostly via software interfaces. The team works in shifts to make sure the medicine reaches clinical trial participants on time.
The analytical imaging team at the Novartis Campus in Basel utilizes state-of-the-art microscopes and microanalysis platforms. During technical development, all drug candidates of Novartis pass through this high-precision “eye” to elucidate minute attributes, including substance crystal form, behavior and chemical solid state characteristics. This also enables the TRD team to identify the underlying characteristics that may contribute to technical challenges at all stages within the research, development and commercial continuum.
The Drug Product Analysis Team is located in the Physics Garden building.
Each product undergoes extensive testing with a variety of methods. Dissolution testing for drug products is a key method to assess the quality of a drug product.
A specialized Analytical & Research Development (ARD) lab in TRD on the Novartis Campus in Basel takes care of the method development for the final drug product.
As the health authorities, such as the FDA and the EMA, have established clear expectations for drug product quality, one being the dissolution of orally administered drugs, the TRD analytics lab evaluates, among others, the time needed until a drug is dissolved in a test liquid that imitates the conditions of a human stomach.



