Science
00
Translating the cycle of life
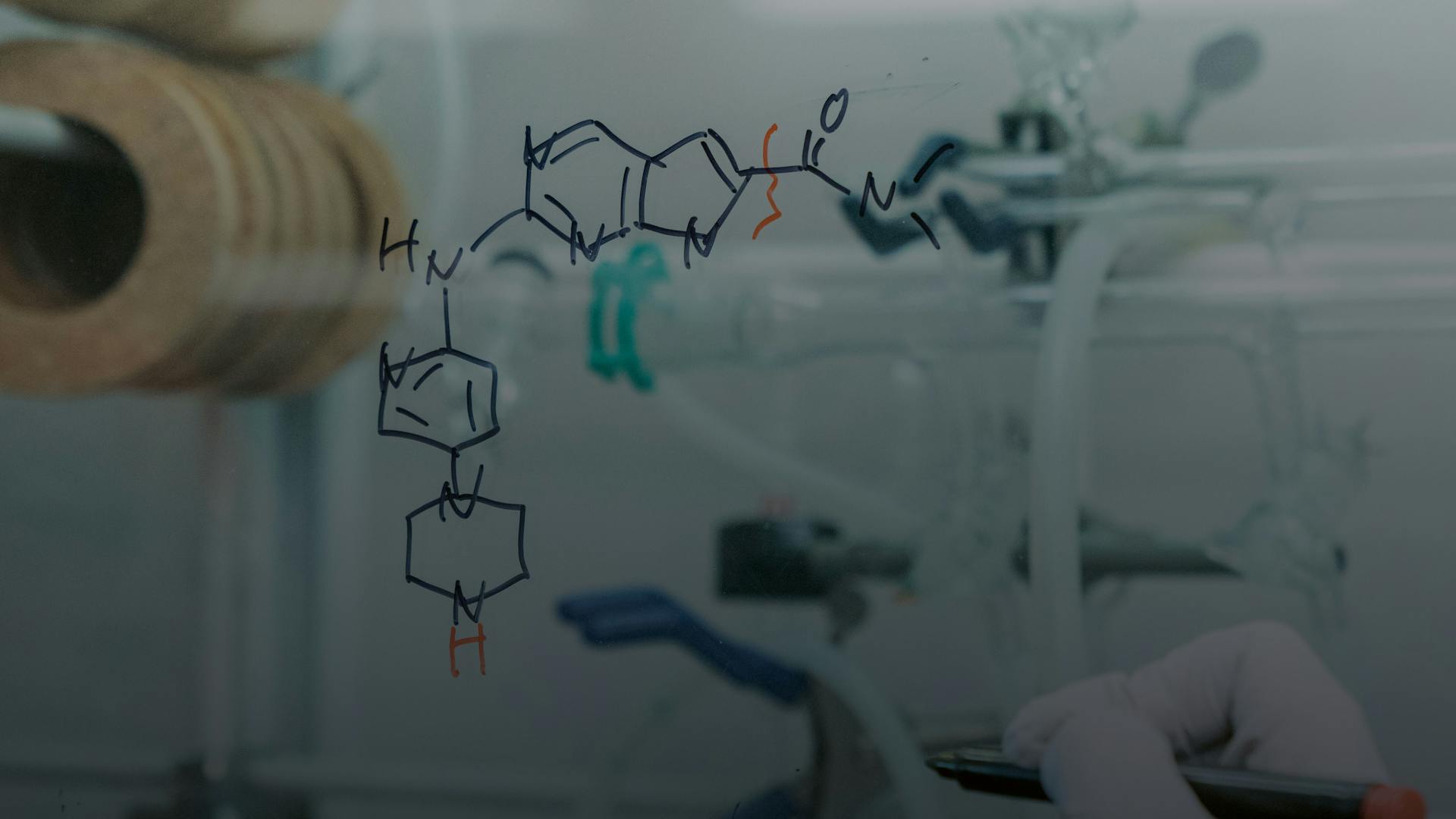
Based on discoveries dating back to the 1970s and 1980s, when academic researchers started to delve into cell division mechanisms to elucidate the cycle of life, Chris Brain from Novartis Biomedical Research and his team translated these fundamental findings into a new medicine. Nearly 10 years later, their breakthrough medicine is helping a growing number of patients suffering from breast cancer.
Text by Goran Mijuk, photos by Andy Kwok


en
de
zh
jp
Share

Live Magazine
This site is intended for a global audience.

en
de
zh
jp
Share


