
A step towards accessible gene therapies
Novartis is collaborating with the Bill & Melinda Gates Foundation to develop an in vivo sickle cell disease gene therapy with the aim to make it widely accessible to patients living in all parts of the world, including in low- and middle-income countries. Besides potentially creating a new cure for sickle cell disease, the ambitious project could help pioneer new access models for gene therapies in global settings.
Text by Goran Mijuk and Michael Mildner, photos by Nana Kofi Acquah and Ernest Ankomah



Once the domain of a few specialized clinics, gene therapies have emerged as an alternative platform to small-molecule and biologic drugs in the pharmaceutical industry over the past decade, with more than 10 approved therapies in the United States alone. But despite their growing popularity, gene therapies are still extremely difficult to discover and develop. Furthermore, they are often expensive to make and mostly focused on the treatment of rare or monogenic diseases that affect only a small number of patients.
While thousands of scientists worldwide are learning how to reduce development timelines and costs, efforts to develop gene therapies for indications affecting more patients with limited financial means have so far been rare. But a recent attempt by the Gates Foundation and Novartis is aimed at changing that. One area of focus is sickle cell disease, a difficult-to-treat genetic blood disorder that is most common in Sub-Saharan Africa. “The goal is to be able to develop the gene therapy in such a way as to make it much more accessible,” said Sue Stevenson, former Executive Director in the Hematology space at Novartis Biomedical Research, who until recently was leading the discovery project from the Novartis side. “This would allow us to reach affected individuals in low- and middle-income settings.”
The need for the development of new treatments for sickle cell disease is urgent, as millions of people throughout the world are severely affected by this life-threatening condition, which causes chronic debilitating symptoms, including acute painful episodes, anemia, chronic pain and fatigue. Ongoing vascular damage and repeated injury to blood vessels and organs also have an extreme physical, financial and emotional impact on patients and their families. This is especially the case in Sub-Saharan Africa, where more than 300000 children are born with the disease each year.
Sickle cell disease is one of the oldest hereditary conditions in the world. It affects around 20 million people worldwide. Most of them, almost 80%, live in Sub-Saharan Africa. Several treatments have been developed to treat the condition, while work on novel gene therapies is also gaining traction. But so far, many of the treatments are out of reach for patients in poorer regions. The collaboration between the Bill & Melinda Gates Foundation and Novartis aims to develop a gene therapy that could also be delivered to underserved populations in Sub-Saharan Africa and elsewhere.
In vivo gene therapy
“The odds to develop an in vivo therapy have never been better,” Stevenson, a gene therapy pioneer with more than 30 years of experience in the field, said. Several decades ago, it was found that some sickle cell disease patients who have higher levels of fetal hemoglobin – a type of globin protein that is in their red blood cells – have a lower disease severity than patients who have a reduced level of this kind of hemoglobin.
This was a key discovery that set the stage for the approach of Stevenson’s team to gene therapy for sickle cell disease. The following insight was crucial: Special proteins such as BCL11A help turn specific genes “on” or “off” by binding to nearby DNA. It was also found that BCL11A, which is a so-called transcriptional repressor, is the master regulator of the switch from fetal hemoglobin to adult hemoglobin. If this switch turns on, adult hemoglobin comes on. “So,” Stevenson said, “if we can prevent BCL11A from binding to the genome, or disrupt the transcriptional repressor itself, then there can be a reversion from adult to fetal hemoglobin.”


Researchers at the laboratories in Uganda and Tanzania, ...
... where Novartis aims to work on its sickle cell project with the Gates Foundation.
The research findings, which Novartis had made originally while working on an ex vivo sickle cell gene therapy, are also applicable to in vivo treatments, which Novartis aims to develop together with the Gates Foundation. In contrast to ex vivo treatments, the in vivo therapies are directly administered to the patient and therefore require less effort in distribution and administration.
If successful, the new gene therapy would add further clout to the efforts of Novartis in this domain. Since its first gene therapy push, which focused on so-called CAR-T therapies (short for chimeric antigen receptor T-cell therapy), Novartis has not only broadened its foothold in the gene therapy field but has also moved into other arenas. These include nuclear medicine and RNA-based therapies, which have become more widespread since the pandemic, when millions of people received RNA-based vaccine shots.
Collaboration with the Bill & Melinda Gates Foundation
Before starting its collaboration with Novartis, the Gates Foundation had already been working on in vivo gene therapies for HIV and sickle cell disease. The program was led by Mike McCune, Head of the Gates Foundation’s HIV Frontiers program, who was asked by Bill Gates in 2018 to set up a platform for bringing curative interventions for HIV to resource-limited parts of the world.
McCune also included sickle cell disease into the program. “Extending the program to create in vivo gene therapies for sickle cell disease made sense from the scientific and population health point of view,” McCune said. “Yet, it was clear up front that the goal of delivering a safe, effective and accessible in vivo gene therapy was highly aspirational and would only be achieved in partnership with others,” McCune said.
In 2019, the Gates Foundation first formed a collaboration with the National Institutes for Health to work on in vivo gene therapies for both HIV and sickle cell disease. Later, it joined forces with Novartis. “Given the work that Novartis was doing in the general field of gene therapy and in the particular area of sickle cell disease, not to mention its ability to bring novel therapies to the clinic, the Foundation reached out to Novartis as well,” McCune explained.
For Novartis, the partnership makes strategic sense. “The Gates Foundation, like us, believes that generating a gene therapy cure for sickle cell disease would help meet a major unmet need and could unlock new opportunities to develop gene therapies for other diseases,” Stevenson said, explaining the rationale behind the collaboration.
The rationale for the partnership is also strong from a scientific perspective. “The reason for this is that the same technology and innovations that we at Novartis are developing for a curative gene therapy for sickle cell disease can be applied to other disorders which require modifications to stem cells,” Stevenson added.
Setting up the collaboration
After Novartis and the Gates Foundation agreed in principle on the collaboration, Novartis applied for a grant agreement with the Foundation in 2020. But things got off to a slightly rocky start because of the pandemic. “We put the initial proposal together, started talking to the Gates Foundation, and decided to do a first face-to-face meeting in March 2020 in Seattle,” Stevenson recalled. “We had purchased our plane tickets already but had to cancel the flights because of Covid shutdowns earlier that same month. That’s how the planned face-to-face meeting turned into my very first Zoom meeting.”
Over the course of 2020, the scope and the aims of the research collaboration proposal were refined. Finally, in the fall of 2020, Novartis entered into a grant agreement with the Bill & Melinda Gates Foundation with the shared goal to discover and develop a novel in vivo gene therapy to cure the clinical manifestations of sickle cell disease.
As part of the agreement, the Gates Foundation is providing funding to offset the costs of the research staff and program costs while Novartis is providing an in-kind contribution of goods and services. As part of the effort, Novartis also entered into an agreement with US-based Precision BioSciences to design a protein that can cut DNA at specific sites, which would allow for the insertion of a functional gene to address the genetic mutation that causes red blood cells to become sickle-shaped.
To set up the clinical research, Novartis scientists have traveled to Tanzania and Uganda to start a collaboration with local sickle cell centers. The aim is to create a network of researchers and clinicians able to pursue lab research and clinical trials to accelerate the development of a novel gene therapy. Thousands of patients and their caregivers would be able to benefit from a potential cure given the high disease burden of the condition, which can lead to stroke at a very early age, among others.
Ensuring access
Besides the scientific aspects, the question of how to ensure equitable access to the treatment is another key priority of this project. “This is a pioneering program in many ways,” said Jonathan Spector, Head of Global Health Strategy and Access at Novartis Biomedical Research. “There are few, if any, precedents for applying such an advanced drug discovery technology, state-of-the art in vivo gene therapy, to specifically address a disease that is most prevalent in resource-limited parts of the world. And the opportunity to do this work as part of a major research collaboration with the Gates Foundation helps to further enable its chance of success.”
Spector acknowledges the access hurdles and explains the team’s strategy: “We at Novartis have the benefit of a “bench-to-bedside” approach for access planning in Global Health through which we start planning early for access in parallel with R&D. We are already leveraging this experience and beginning to plan for access from the outset of research.”



Sue Stevenson during her research journey in Uganda and Tanzania.
Jonathan Spector, Tovy Ayala and Serena De Vita during their research journey in Uganda and Tanzania.
Setting up a joint research program involving partners across borders requires major planning efforts.
The grant agreement also includes specific provisions in support of the Gates Foundation’s global access policies, which ensure access to any resulting products and innovations to people who need them most within developing countries.
Novartis, meanwhile, has also started to work with patient groups, physicians, policy makers and scientists in Tanzania, Uganda and Ghana. “The medicine we discover will need to be effective and safe in populations where the disease is highly endemic,” Spector explained. “To achieve that, we will work closely with sickle cell disease communities around the world, including in Africa. Through this program we will also have the privilege to host some of our African scientist colleagues in our labs in Cambridge and Basel to work shoulder to shoulder.”

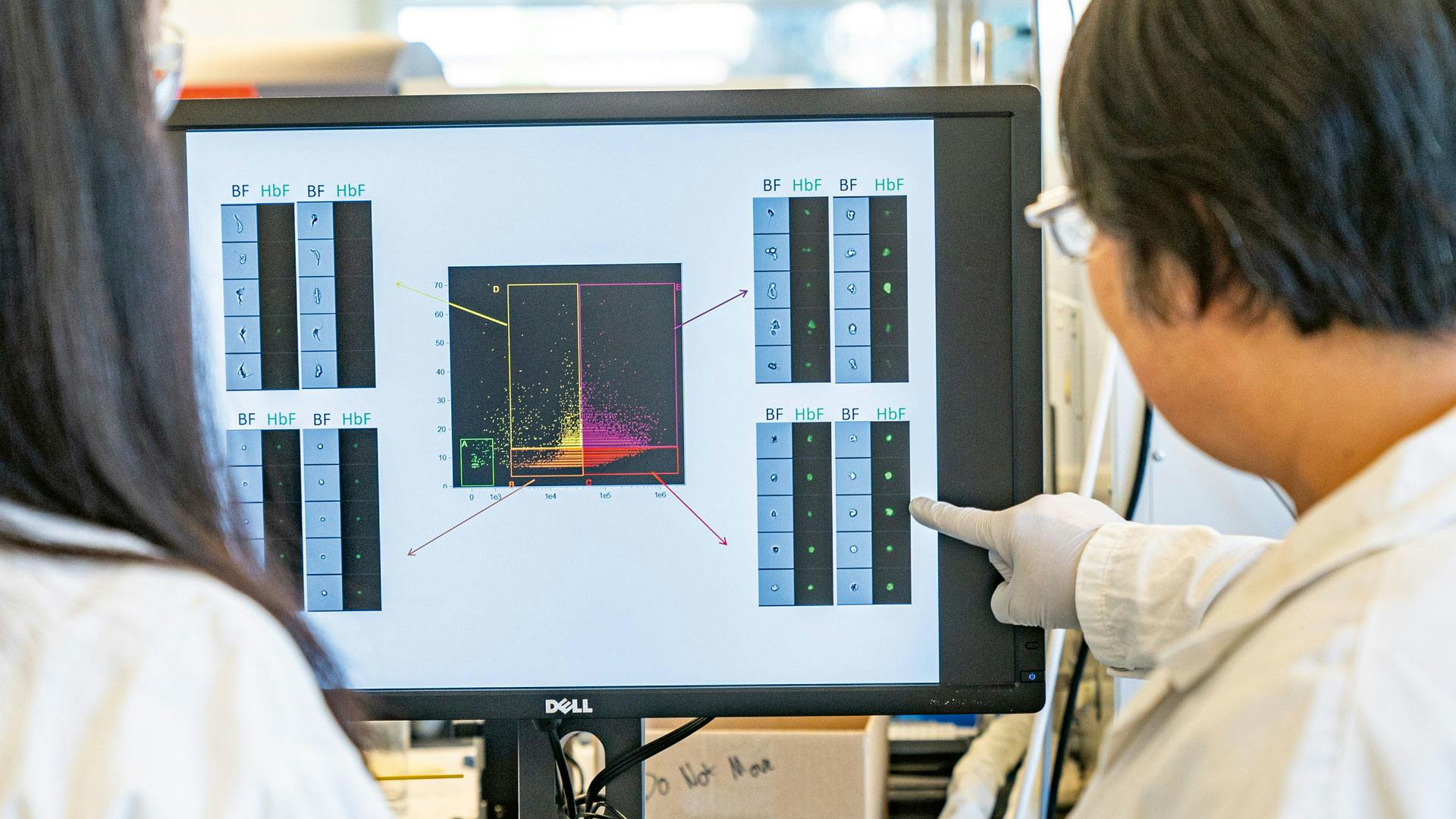
Researchers in Cambridge already work on a potential sickle cell gene therapy.
Scientists discuss new data points coming out of the recent tests.
Broader program
The Novartis in vivo gene therapy efforts are part of the company’s broader sickle cell disease program, which it started several years ago and initially centered on supporting efforts in Ghana. Since then, Novartis has implemented a comprehensive approach to disease management through public-private partnerships in several African countries and is aiming to develop a series of public health initiatives, which include early-intervention strategies, such as screening and diagnosis.
This also includes a portfolio of digital initiatives. For example, as part of the program, Novartis supported the Sickle Cell Foundation of Ghana in the development and roll-out of a digital phone-based application to facilitate the collection and management of data in the country’s national newborn screening program. To date, more than 90000 babies have been registered in the app, which is being expanded to other countries in Africa.
“All of these efforts are crucial to change the disease burden and bring change to patients,” Stevenson added. “If we could bring safe and effective gene therapies to market that target and eliminate the root cause of the disease, this could be life-changing for millions of people suffering from sickle cell disease and another big victory for medicine.”
Furthermore, if Novartis succeeds in its efforts to develop an in vivo gene therapy, such a breakthrough could also initiate a new phase in the history of gene therapies, which to date have been only administered to a few patients because of the high costs, complexity and difficulty of administration.
An in vivo gene therapy for sickle cell disease could help change that for good.





