Live. Magazine
Researchers often test millions of molecules before finding any that interact with a drug target. Discovering these sought-after molecular structures – called hits in the industry – is complex and resource-intensive. It is also just one of the first of many steps in the complex pharmaceutical research and development process.
Hits are starting points that give research teams clues about where to go next – for example a rough idea of the right shape the molecule needs. From there, chemists have thousands of chemical building blocks that they can swap in and out to change the molecule’s properties until they finally have a drug candidate they can test in clinical trials.
“It’s a little like building with Legos,” says Pascal Furet, a molecular modeling specialist. “We have a toolkit of chemical building blocks that we can use to improve the activity of the hit, as well as how the molecule behaves in a cell or in the body.”
With close to 30 years of experience each, Joseph Schoepfer and Pascal Furet are both experts in this stage of drug discovery. Schoepfer leads a team of medicinal chemists who synthesize hundreds of new molecules – variations of a hit – to identify the chemical features that give a drug candidate the best biological properties. Furet complements Schoepfer’s medicinal chemistry teamwork with computational predictions and recommendations for prioritizing which molecules to synthesize.
This close collaboration between the fields of medicinal chemistry and computer-aided methods is a cornerstone of so-called structure-based drug design, which emerged in the 1980s. Many diseases can be tracked back to malfunctioning proteins, and structure-based approaches use protein structures to create drugs that fit exactly into the binding pockets of protein drug targets.
The right properties
Early drug screening frequently uses biochemical assays, which are tests with simplified conditions that measure whether molecules inhibit a purified protein target. These tests are advantageous in the early, exploratory phases of drug discovery because they are fast, relatively inexpensive, and they give clear answers about how a molecule and a protein interact when they are together in isolation.
But drugs don’t have the luxury of interacting with their targets in isolation. They need to be fully equipped to work in cells and living organisms – environments that are filled with fluctuating conditions and thousands of other proteins and biological molecules. Drug researchers also have to navigate the complex balance between maximizing a drug’s desired activity and minimizing any toxicity and safety concerns.
But adapting hits for these challenges is precisely the specialty of medicinal chemists.
“Jahnke and Marzinzik were instrumental in paving the way with a molecule that had activity in biochemical and cellular assays, but it didn’t have optimal pharmacological properties yet,” says Schoepfer.
“That’s when we took over the optimization and development of the compound on its path to becoming a drug.”
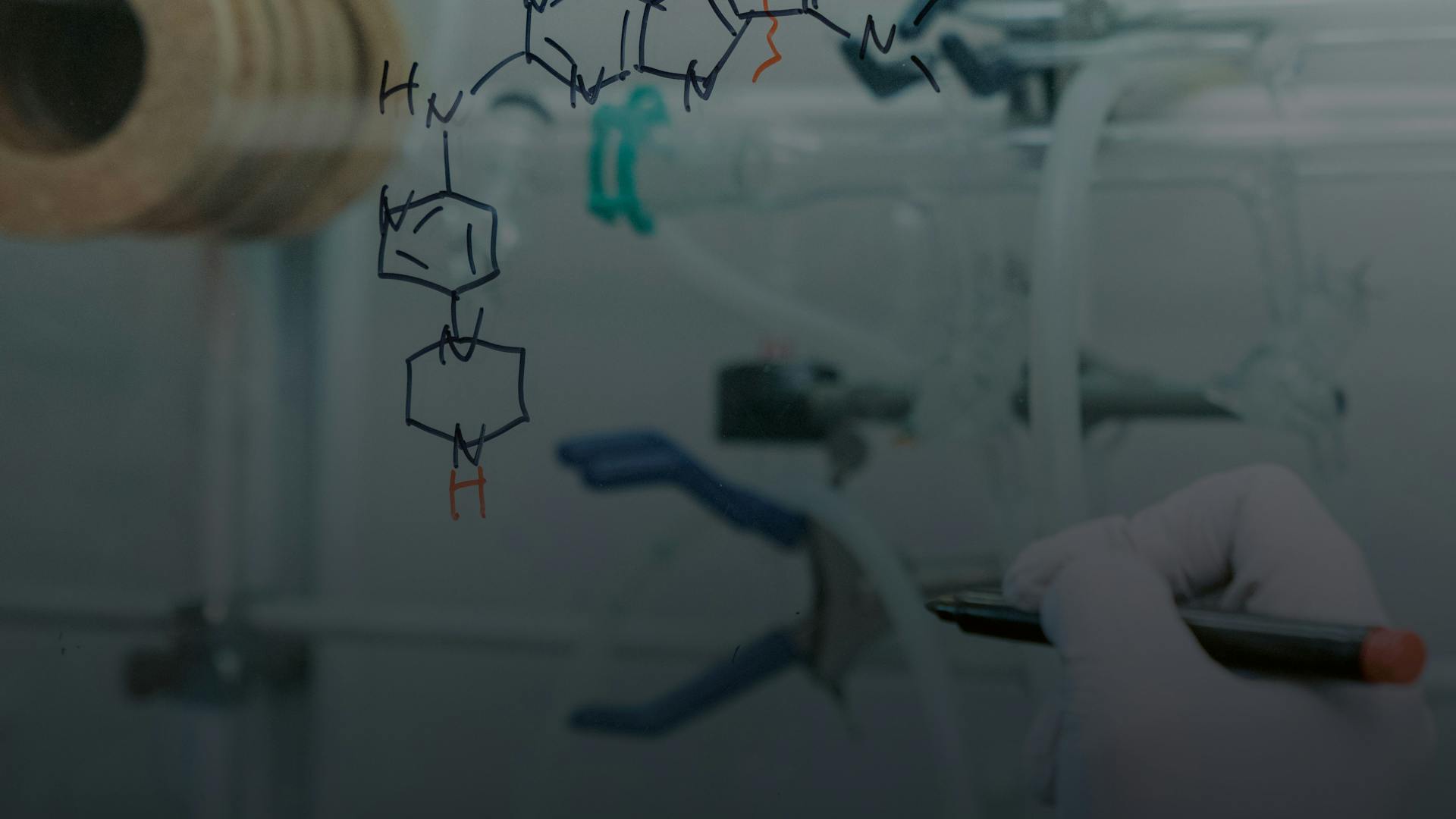
Finding the right molecule shape is akin to an art as chemists have myriads of options to choose from. Mathematics, experience and intense lab work are instrumental for this work.
The field of medicinal chemistry has existed for over a century. The medicinal chemist’s challenge is turning hits into drugs. They have thou-sands of chemical variations and building blocks in their toolkits, along with some knowledge about the impacts those building blocks may have in the human body.
But chemists estimate that there are roughly 10⁶⁰ potentially drug-like molecular structures that could be made, which is more than the number of the atoms in the solar system. That is a far greater number of molecular possibilities than chemists have the time or resources to make – a dilemma which can be helped by computational approaches.
Guided by physics
“Using molecular modeling and the laws of physics, we can compute how well molecules interact with the protein-binding pocket, at least in theory,” explains Furet. “This lets us predict the most beneficial molecular changes and prioritize what we want to synthesize and test in the lab next.”
This also entails close collaboration with the colleagues from the medicinal chemistry. “It’s a very interactive process with input from both sides,” says Schoepfer. In the project on which they worked together, Schoepfer pointed to the regular exchange: “We had regular meetings all together to look at the structures and discuss how we might improve the compounds further.”



A computer model of a protein. The trick is to find a space where a compound can interfere to block a disease pathway.
Joseph Schoepfer: Chemistry is a brain and hands-on science.
Pascal Furet: Drug design also relies on physics.
On the computational side, modeling provides insights about many more molecules than chemists have time to make, which streamlines the process by ruling out the least promising molecules. However, at the same time, there are still many molecules that the field of chemistry doesn’t yet know how to synthesize. Chemists bring essential knowledge about what is actually feasible in the lab.
But the pure modeling approach is nevertheless important and can help identify unusual opportunities that might otherwise be missed. For example, Furet suggested an uncommon chemical building block that turned out to be one of the key modifications that helped increase the potency of the final drug molecule.
“I made a suggestion based on the physics and the possibility of using a slightly larger atom to completely fill the binding pocket – but from a chemist’s perspective that building block is almost never used in drugs,” says Furet.
3D structure of a protein therapeutic target obtained by X-ray crystallography.
“The team wouldn’t have considered that modification if my calculations hadn’t shown that it might be very beneficial – but once they synthesized and tested it, it was a big improvement to the molecule.”
“Other researchers in the field are still amazed that we used that chemical group in the final drug – and that it works so well,” adds Schoepfer.
The combination experiment
“This process is still a kind of art,” says Furet. “Even with the best science, the interactions between a small molecule and a protein are very complex and we’re not yet at the level of accurately predicting all of the possibilities, but we had a very good candidate by the end.”
Schoepfer, Furet and their colleagues spent close to two years optimizing the hit that Jahnke and Marzinzik had handed over to them, synthesizing several hundred variations along the way. In the end, they had a drug candidate that is one of the highlights of their careers. This molecule might also help overcome one of the biggest challenges in oncology.
Since Jahnke and Marzinzik began the project, one of the final goals had been to develop a combination treatment that could prevent the emergence of resistance – one of the most significant limitations in successfully treating cancers of all types. Their idea was to combine the new drug with the earlier-generation drugs, which operated through a different binding pocket. The hypothesis was that this two-pronged strategy might effectively eliminate resistance. When they finally tested this idea in the lab, it worked better than any of them had imagined it would.
“When our biologist collaborators sent those preclinical data, those were really the most impressive scientific results I have observed during my career,” says Schoepfer. “There was no relapse when they used the combination – it was a breakthrough.”
Since then, the new drug has been progressing through clinical trials, both on its own and in combination, where it is providing some of the first evidence that it might be possible to eliminate drug resistance for some cancers. “This drug is first in its class and it’s helping people who have run out of other options,” adds Furet. “For a drug researcher, that’s really the achievement we aspire to.”